how to draw molecular orbital diagram for heteronuclear molecules
We must construct molecular orbital diagrams for the three molecules and use the results to interpret the trend. In general a molecular orbital in a polyatomic system extends over all.
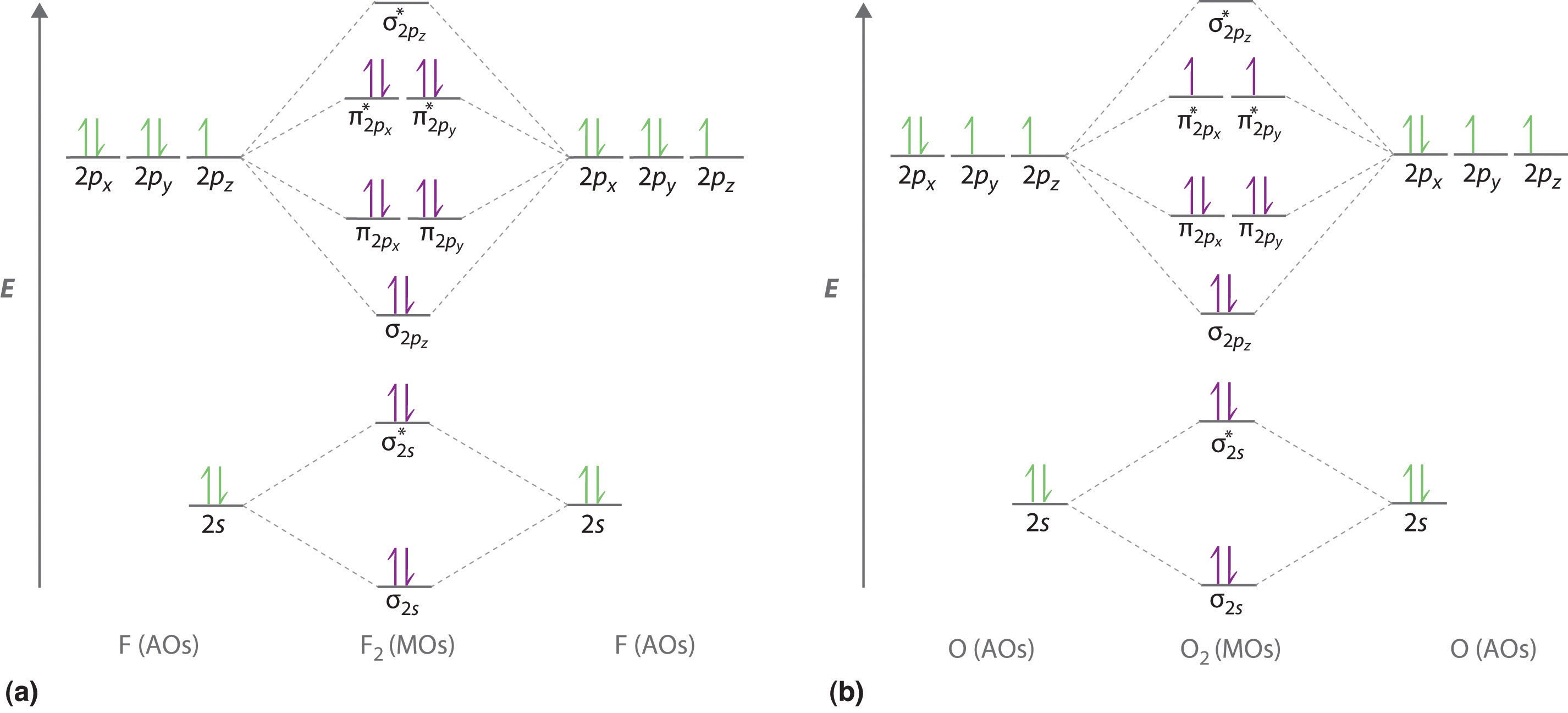
1 1 4 5 Molecular Orbital Theory Chemistry Libretexts
Indeed molecular orbital theory forms the basis for most of the quantitative theoretical investigations of the properties of large molecules.
. In this case were using the standard one. Then well build the MO diagram from scratch. They should not therefore be much affected by the field of the proton or interact significantly with the H 1s orbital.
However it did mention drawing Correlation diagrams for heteronuclear molecules taking into account the electronegativity of the atoms More electronegative atom has lower energy levels. Draw a schematic molecular orbital diagram for the adsorption of a diatomic molecule on a d metal. CHEM 2000 Exercises and Practice Test Questions.
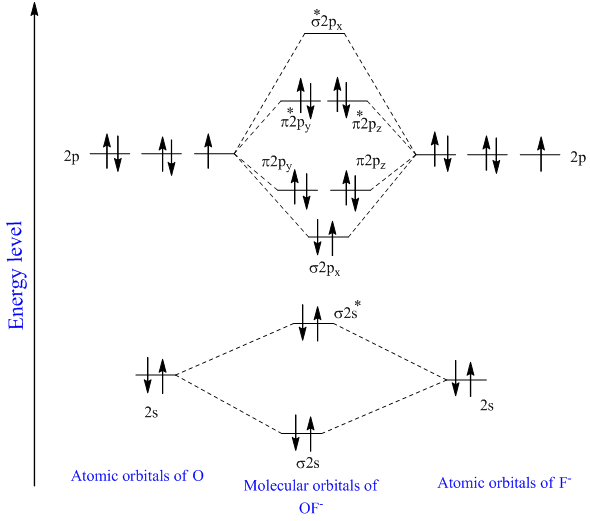
D65 MOs for Heteronuclear Diatomic Molecules. HF nb σ σ Energy H 136 eV 1s F 186 eV 402 eV 2s 2p So HF has one σ bond and three lone electron pairs on fluorine. MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine.
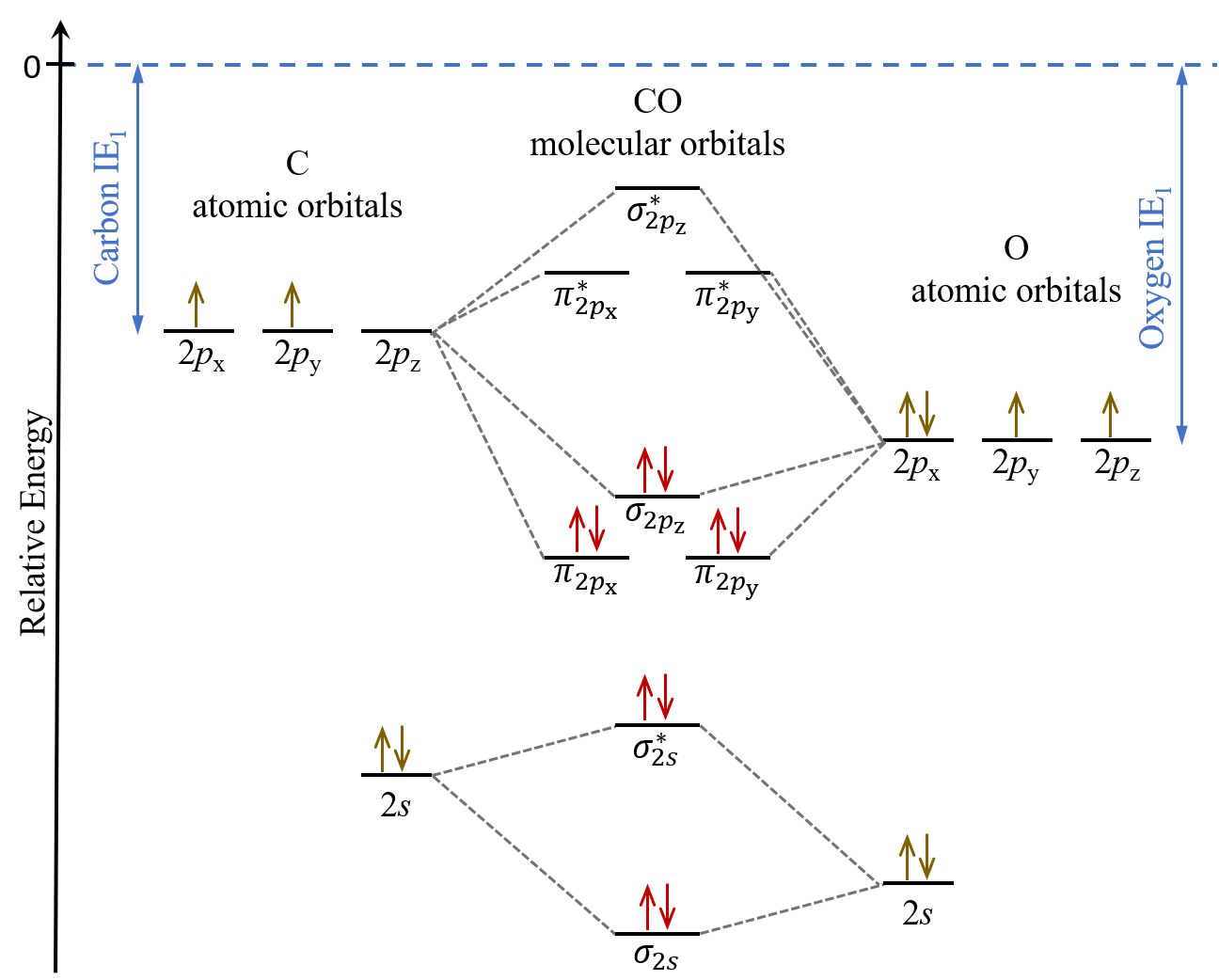
Because the and ˇ orbitals arise from independent LCAOs we can build the ˇ orbital energy diagram independently of the orbitals. The molecular orbital of lowest energy in these molecules the ls molecular orbital should be. 12-12 This video describes the molecular orbital theory diagram of CO placing emphasis on how MO theory differs for homo and heteronuclear diatomics.
The further to the right your element is the lower its energy levels are. Now I know that the more electronegative atoms orbitals are going to be lower in energy than the less electronegative atom. Building Molecular Orbital Diagrams for Homonuclear and Heteronuclear Diatomic Molecules Due to symmetry of the molecule homonuclear MOs are less difficult to derive than heteronuclear molecules and polyatomic molecules.
The head-to-head overlap giving σ molecular orbitals results in greater overlap making its bonding molecular orbital the most stable and lowest energy while the σ antibonding is least stable and has the highest energy Figure 924 Molecular orbital energy diagram for homonuclear diatomic molecules made from atoms of atomic number 8-10. First step is to determine which MO diagram were using. Thats it for the MO diagram of B_2.
Thus the rule becomes. 05A Band Theory and Bonding in Metals. Draw the MO diagram for B_2.
In the course reader. 01 Reviewing Atomic Orbitals Electron Configurations and Lewis Diagrams. These however also have their own depth.
Label each level with σ σ π π In the construction of molecular orbital diagrams for heteronuclear molecules the bonding mos are shown closed to electronegative atoms while. However with more atoms computers are required to calculate how the atomic. AO2px AO2px π2px π 2px weak sidelong overlap AO2py AO2py π2py π 2py weak sidelong overlap AO2pz AO2pz σ2pz σ 2pz strong head-on overlap Thus we take 10 atomic orbitals and generate 10 molecular orbitals in.
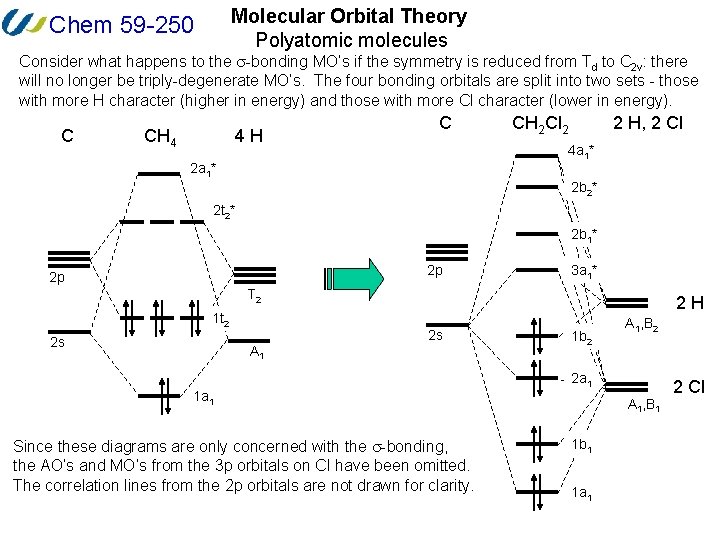
05B Valence Bond Theory. Creating molecular orbital diagrams for molecules with more than two atoms relies on the same basic ideas as the diatomic examples presented here. Boron has 2 electrons in the 2s orbitals and 1 electron in the 2p orbital.
04 Molecular Orbitals of Polyatomic Molecules. The drawing of the Mo diagram of heteronuclear molecules is quite different from the drawing of the mo diagram of homonuclear molecules. How to Draw Molecular Orbital Diagrams MO DIAGRAMS Explanation - YouTube.
02 Molecular Orbitals of Homonuclear Diatomics. I have learned so far that there are two ways to set up MO diagrams for homo-nuclear diatomic molecules. Draw the molecular orbital diagram for the oxygen molecule O 2.
Diagrams like these change according to if youre working with homonuclear molecules - molecules made of one element - or heteronuclear molecules - molecules made of different elements. 03 Molecular Orbitals of Heteronuclear Diatomics. One was with the pi 2p bonding MO below the σ2p bonding orbital and the reverse for atoms with an atomic number 8.
Draw tiny updown arrows for each electron that the atom possesses overall. 44 Molecular orbital diagram for octahedral. Diatomic molecules with two non-identical atoms are called heteronuclear diatomic molecules examples include CO NO and HCl.
You should know this from General Chemistry all three 2ps are degenerate. Molecular orbital diagrams are diagrams of molecular orbital MO energy levels shown as short horizontal lines in the center flanked by constituent atomic orbital AO energy levels for comparison with the energy levels increasing from the bottom to the top. Because the and ˇ orbitals arise from independent LCAOs we can build the ˇ orbital energy diagram independently of the orbitals.
The F 2s is nonbonding. The concept of a molecular orbital is readily extended to provide a description of the electronic structure of a polyatomic molecule. Involving heavier atoms makes it harder to guess at molecular orbital diagrams and there is need for quantum chemistry calculations.
The 1s orbital energies of Li C and F all lie well below that of the H 1s orbitalThe charge densities of these inner shell orbitals are tightly bound to their respective nuclei. Schematic molecular orbital diagram for heteronuclear first-row diatomics. Molecular orbital diagrams for these molecules have one more layer of complexity but they also serve to explain many bond and molecular properties we will encounter later.
Draw a vertical arrow labeled E for energy on the left side of a piece of paper oriented portrait rather than landscape and draw one horizontal line for each orbital on the energy scale. Lines often dashed diagonal lines connect MO levels with their constituent AO levels. Shields shows you how to draw the MO correlation diagram for cyanide CN- calculate the MO bond order and write the MO electron configuration with an.
Draw out the MO diagram and label in the valence electrons. To make sense of the complexities introduced by antibonding we build molecular orbital diagrams. Indeed molecular orbital theory forms the basis for most of the quantitative theoretical investigations of the properties of large molecules.
Such as H 2O NH 3 and CH 4 However notice the difference between orbitals of homonuclear diatomic for. Drawing the correlation diagram for homonuclear atoms seems pretty straightforward if you follow the atomic charge and valence shell e- rule. From this diagram calculate the bond order for O 2.
Now MO diagrams are only simple for elements of the second row of the periodic table ceLi through ceNe.
Molecular Orbital Theory Diatomic Molecules Heteronuclear Molecules Chem

Molecular Orbital Theory For Heteronuclear Diatomic Molecules Pt 4 Youtube

Molecular Orbital Mo Diagram Of Polyatomic Molecules Beryllium Dihydride Beh2 And Water H2o Youtube
How Would You Describe The Co Bond In Terms Of Molecular Orbital Theory Quora
D6 5 Mos For Heteronuclear Diatomic Molecules Chemistry 109 Fall 2021

Solved Chapter 5 Problem 10p Solution Inorganic Chemistry 5th Edition Chegg Com

Molecular Orbitals Molecular Orbitals For Homonuclear Diatomics

Molecular Orbital Diagram Of Polyatomic Co2 Molecules Chemical Bonding Molecular Structures Youtube
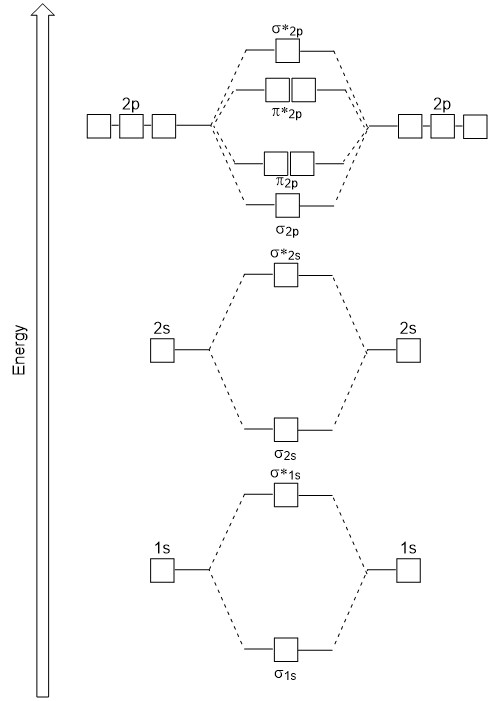
8 Drawing Molecular Orbital Diagrams Flux Science

Solved Draw A Molecular Orbital Diagram For Each Of The Chegg Com

1 1 4 5 Molecular Orbital Theory Chemistry Libretexts
Molecular Orbitals Introductory Chemistry 1st Canadian Edition Clone

Molecular Orbital Theory For Homonuclear Diatomic Molecules Pt 3 Youtube

Molecular Orbital Theory Heteronuclear Diatomic Cyanide Cn Example Youtube
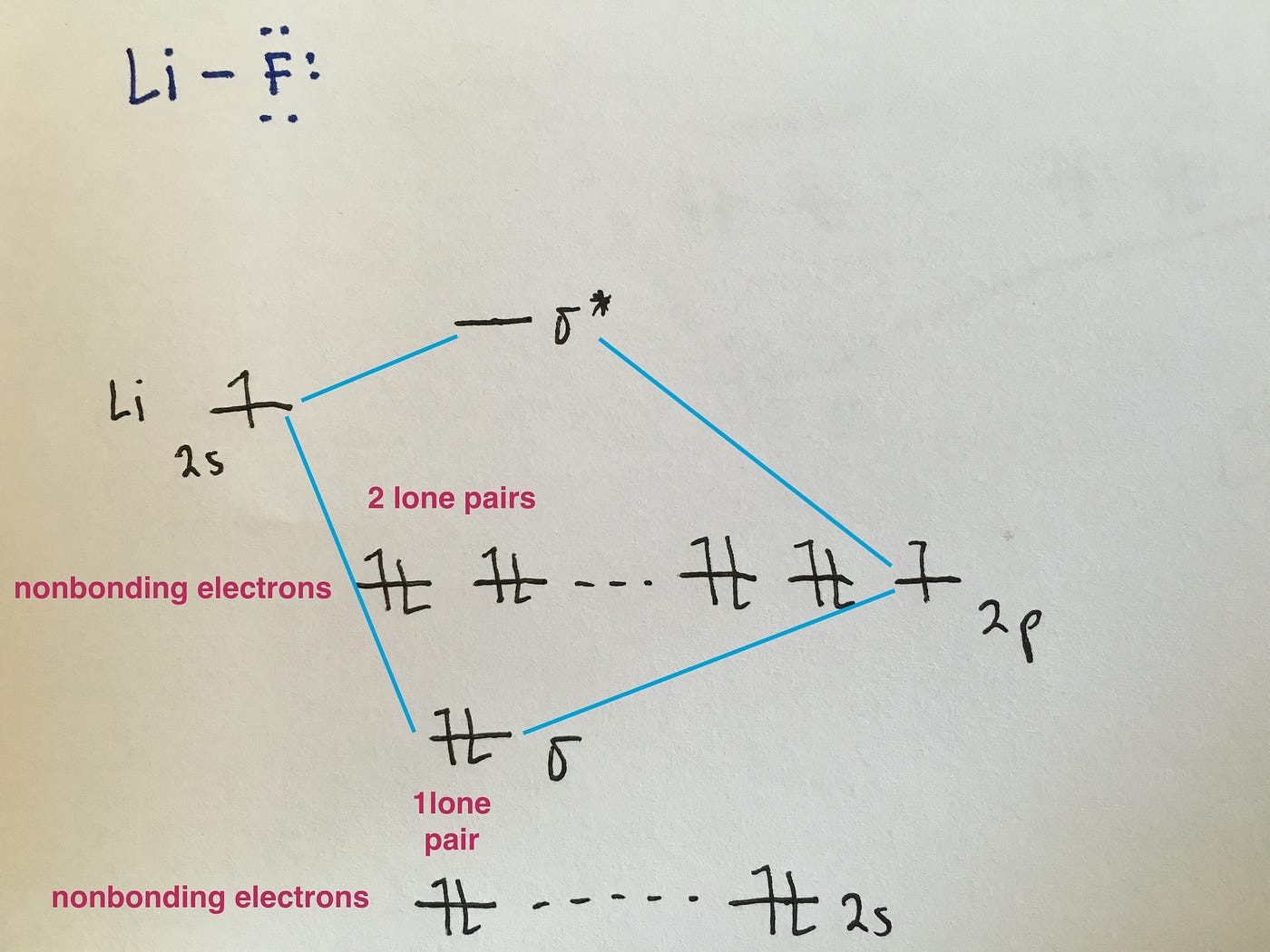
Molecular Orbital Diagrams Simplified By Megan A Lim Medium
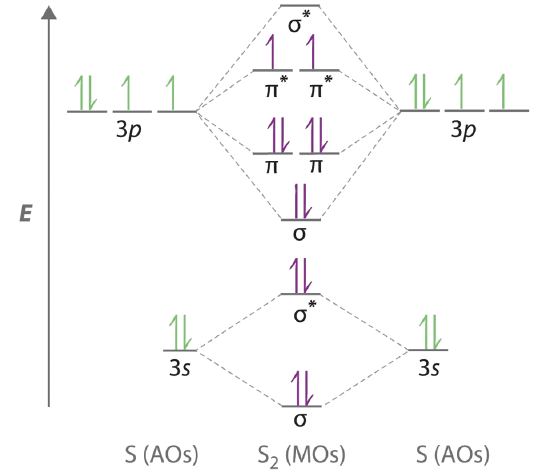
9 8 Second Row Diatomic Molecules Chemistry Libretexts
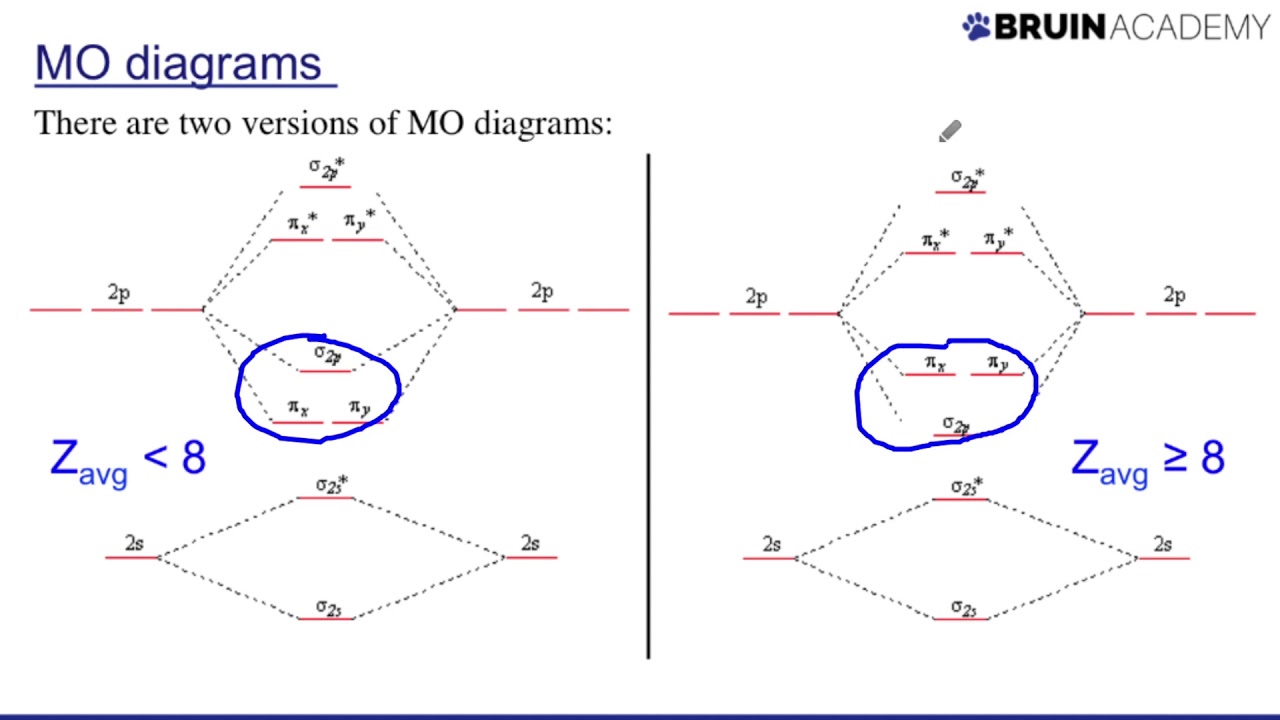
Drawing Molecular Orbital Diagrams Youtube
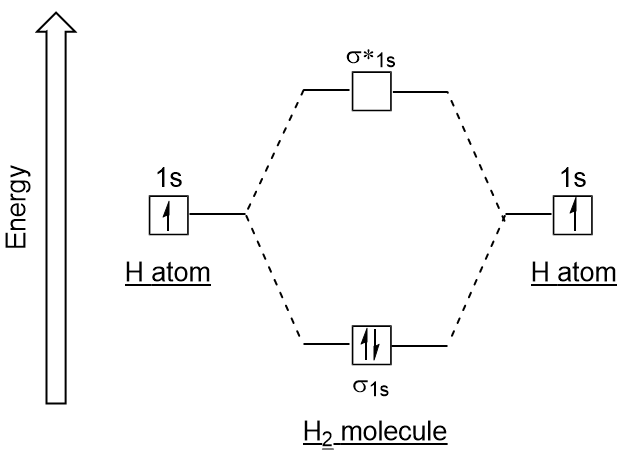
Molecular Orbitals Introductory Chemistry 1st Canadian Edition